Page 6 - HFA_Dateline_2019_Q1_Spring_SpecialEdition
P. 6
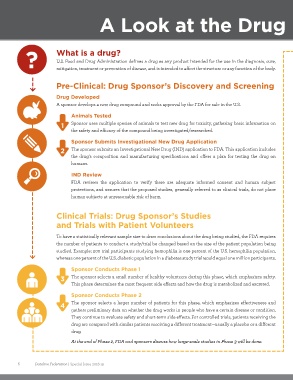
A Look at the Drug
? What is a drug?
U.S. Food and Drug Administration defines a drug as any product intended for the use in the diagnosis, cure,
mitigation, treatment or prevention of disease, and is intended to affect the structure or any function of the body.
Pre-Clinical: Drug Sponsor’s Discovery and Screening
Drug Developed
A sponsor develops a new drug compound and seeks approval by the FDA for sale in the U.S.
Animals Tested
1 Sponsor uses multiple species of animals to test new drug for toxicity, gathering basic information on
the safety and efficacy of the compound being investigated/researched.
Sponsor Submits Investigational New Drug Application
2 The sponsor submits an Investigational New Drug (IND) application to FDA. This application includes
the drug’s composition and manufacturing specifications and offers a plan for testing the drug on
humans.
IND Review
FDA reviews the application to verify there are adequate informed consent and human subject
protections, and assures that the proposed studies, generally referred to as clinical trials, do not place
human subjects at unreasonable risk of harm.
Clinical Trials: Drug Sponsor’s Studies
and Trials with Patient Volunteers
To have a statistically relevant sample size to draw conclusions about the drug being studied, the FDA requires
the number of patients to conduct a study/trial be changed based on the size of the patient population being
studied. Example: 200 trial participants studying hemophilia is one percent of the U.S. hemophilia population,
whereas one percent of the U.S. diabetic population in a diabetes study trial would equal one million participants.
Sponsor Conducts Phase 1
3 The sponsor selects a small number of healthy volunteers during this phase, which emphasizes safety.
This phase determines the most frequent side effects and how the drug is metabolized and excreted.
Sponsor Conducts Phase 2
4 The sponsor selects a larger number of patients for this phase, which emphasizes effectiveness and
gathers preliminary data on whether the drug works in people who have a certain disease or condition.
They continue to evaluate safety and short-term side effects. For controlled trials, patients receiving the
drug are compared with similar patients receiving a different treatment—usually a placebo or a different
drug.
At the end of Phase 2, FDA and sponsors discuss how large-scale studies in Phase 3 will be done.
6 Dateline Federation | Special Issue 2018-19