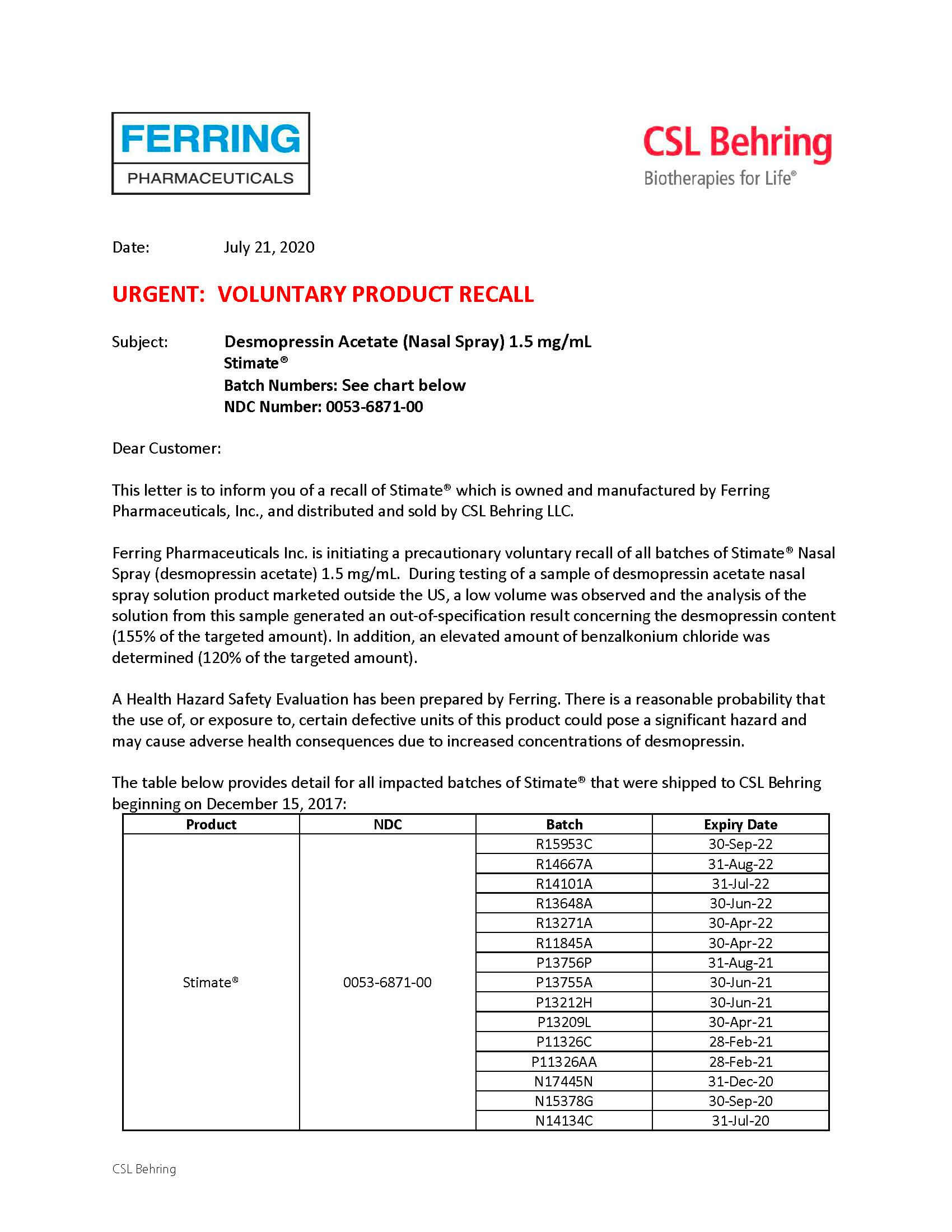
Late today (July 21, 2020), HFA and NHF learned of a pharmacy level product recall of Stimate (desmopressin) nasal spray manufactured by Ferring Pharmaceuticals, and distributed in the U.S. by CSL Behring. Ferring has prepared a Health Hazard Safety Evaluation, warning that “there is a reasonable probability that the use of, or exposure to, certain defective units of this product could pose a significant hazard and may cause adverse health consequences due to increased concentrations of desmopressin.” Patients should not use the product and should contact their physician about changing their treatment regimen. As of mid-July 2020, affected batches of these products have been recalled worldwide.
HFA and NHF recognize that recalls can be very unsettling for many in the bleeding disorders community. We are in communications with CSL Behring to obtain additional detailed and timely information regarding the events that led up to the recall, as well as CSL’s plans for publicizing and accomplishing the recall. As has been our past practice (and consistent with the principles articulated at the January 2020 Safety Summit), HFA and NHF commit to keeping the bleeding disorders community informed as we gather further information.
For the full text of CSL Behring’s announcement, click here.
Please note that NHF and HFA do not recommend, endorse or make any representation about the efficacy, appropriateness or suitability of any specific products, treatments, or opinions. If you have any questions or concerns about your medical treatment, including your use of the product at issue, please consult your physician.