1000
The first description of an inherited bleeding disorder is referenced in the Talmud, an ancient body of Jewish law, compiled in the 2nd century AD.
“An incident occurred where a woman had circumcised her son and he died. Her sister circumcised her son, and he also died. The third sister brought her son before the Rabbi Yohanan, who said, “Go and circumcise your son. Two occurrences is not enough to establish presumption [that the child will die.]”
Rabbi Abaye said to him, “You must be certain that you are accurate, otherwise you may be permitting harm to the child.”
1600 - 1900
1639. The first European with a bleeding disorder arrives in the American colonies.
1791. Isaac Zoll from Virginia dies at age 19 from a minor cut on his foot. He is regarded as the first American with hemophilia.
1803. Hemophilia is first named.
1839. The book Domestic Medicine is published. It includes treatments for hemorrhages and internal bleeding.
1837 - 1901
Queen Victoria ruled the United Kingdom from 1837 until her death in 1901. She is the originator of hemophilia in the European royal families.
The 20th Century


1926. von Willebrand Disease is first described.
1943. Advances in transfusion medicine improve life expectancy for someone with severe hemophilia to 20 years.
1948. The National Hemophilia Foundation is founded by Betty Jane and Bob Henry, parents of a son with hemophilia.
1949. The U.S. Army shows that soldiers using pooled plasma have a higher incidence of jaundice.
1957. Since treatment is limited to whole blood, researchers seek alternative means to prevent and treat bleeding episodes.
1959. Mary M. Gooley assembles one of the first comprehensive care centers in Rochester, NY.
1960. This era marks a time of research into effective treatment for hemophilia as whole blood continues to be the only effective treatment.
1963
The World Federation of Hemophilia (WFH) is founded by Frank Schnabel, a Canadian with severe hemophilia A. The first WFH Congress was held in Copenhagen on June 25, 1963, and was attended by representatives from 12 different countries.
Photo of WFH in Paris, 1965
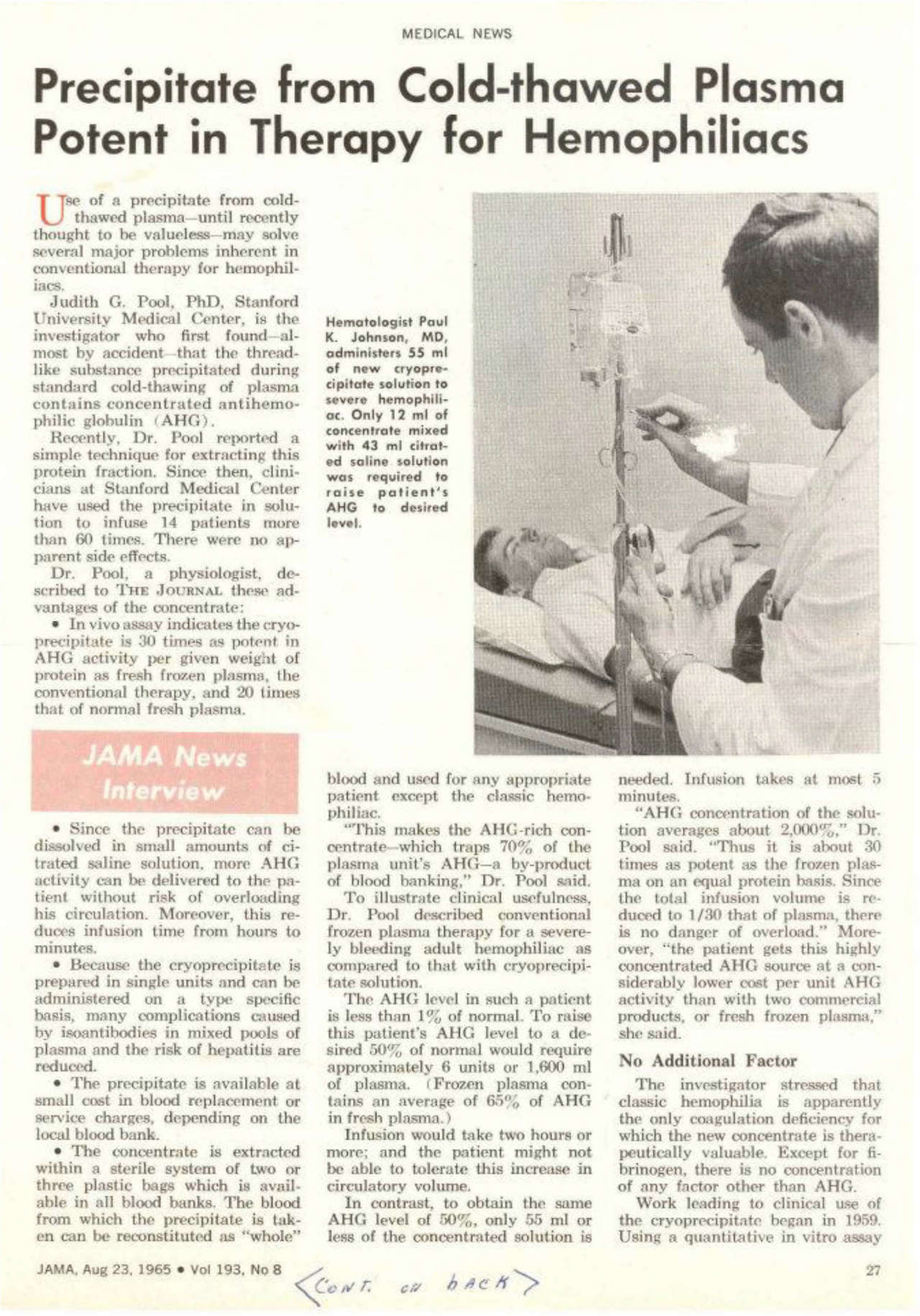
1965. Dr. Judith Graham Pool discovers cryoprecipitate. This is the first successful treatment for hemophilia beyond whole blood. After cryoprecipitate is developed, life expectancy grows to 24 years old.
1967. Working at Hyland Labs, Dr. Brinkhaus discovers a method to distill cryoprecipitate into clotting factor, an even more potent therapy for controlling bleeding.
1968. As factor concentrate becomes available, patients are given instructions on how to access it. Availability of factor concentrate varies by center and region.
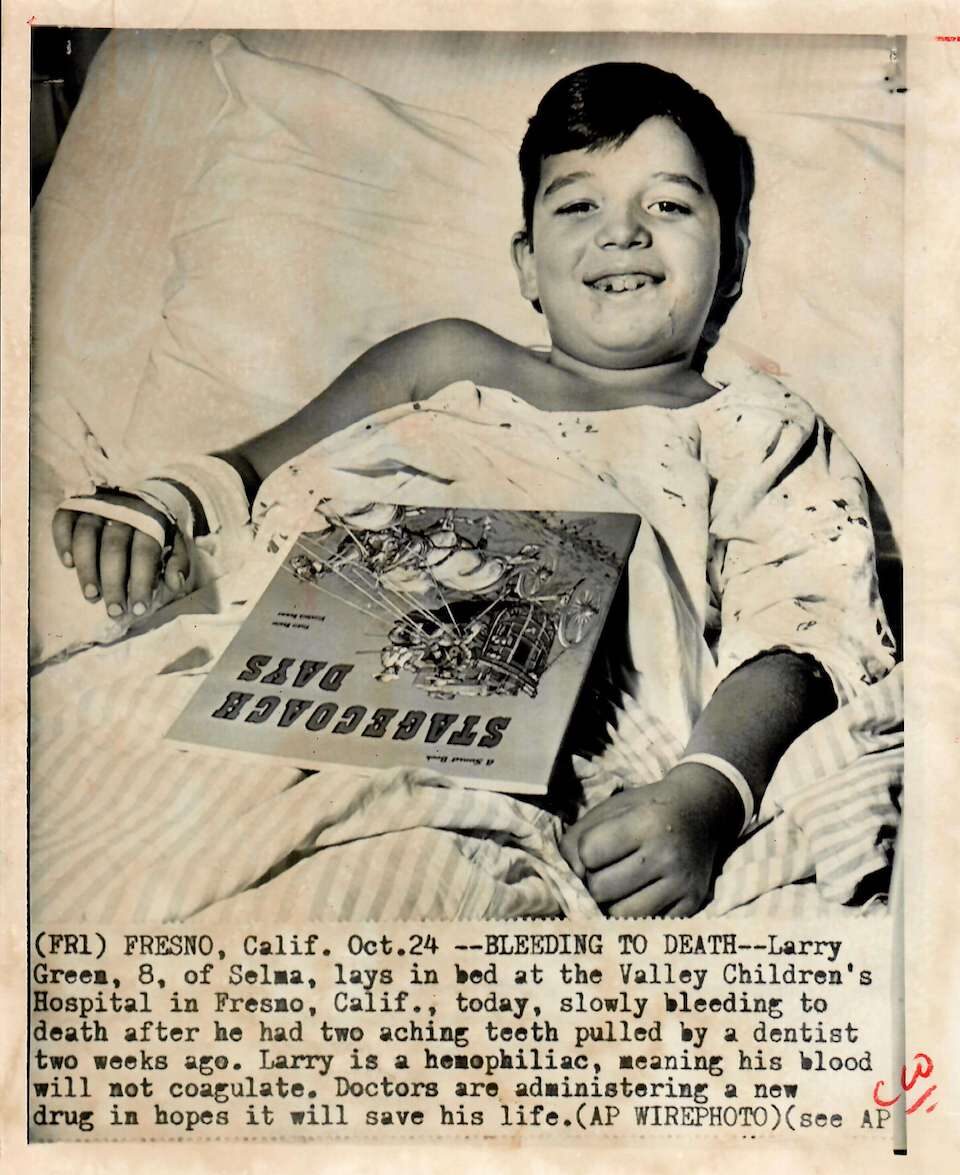
Women, people of color, and those living in rural areas are rarely mentioned in media descriptions of people with bleeding disorders.
1969
Camp Bold Eagle in Michigan becomes the first hemophilia summer camp in the country for children with bleeding disorders. Camp gives children the opportunity to be around others like themselves, and for many this is the first time they meet others with hemophilia.



The 70s
1970. Despite medical advancements, many patients with hemophilia have joint damage from years prior. The community rallies support to help these patients receive appropriate care.
1971. Factor concentrate usage continues to vary. Some doctors are still prescribing and instructing patients to use cryoprecipitate in the early 1970s. For others, treatment at home is becoming more prevalent and patients no longer feel tethered to the hospital.
1972. For decades, hemophilia families have organized blood drives to replace the donations needed for transfusions. These remain common, despite the increasing availability of cryoprecipitate and clotting factor concentrates.
1973. The Hemophilia Act passes, launching the formation of a nationwide network of federally-funded hemophilia treatment centers.
1974. Hepatitis non-A and non-B, later known as hepatitis C, is a growing concern due to pooled blood being used in treatment. However, it is deemed an “acceptable risk” since clotting factor extends the patient life expectancy.
1975. The media continues to draw attention to the plight of those living with hemophilia and other bleeding disorders, highlighting that these conditions can affect many generations within one family. Even with advances in treatment, factors such as race, ethnicity, gender, socioeconomic status, and location create persistent barriers to treatment.
1977. An article in the Journal of the American Medical Association notes it is possible to heat treat albumin, a blood product, to effectively kill some of the hepatitis viruses. While this process was industry standard in Germany, another major producer of antihemophilic factor concentrates, it did not become mainstream in the U.S. until the mid-1980s.
1978-1979. Those living with hemophilia now enjoy an improved quality of life.
We were called bleeders back then. I credit the fact that I’ve done so well, as far as physically, because my parents did allow me to do things. Sometimes I’d still get bruises and stuff, and I’d have to get treatment. I missed a lot of school.
Felix Louis Garcia
The 80s
June 1981. The U.S. Centers for Disease Control (CDC) reports a rare pneumonia in five young men in Los Angeles. Increasing reports of compromised immune system conditions among gay men lead health officials to call the outbreak Gay-Related Immune Deficiency, or GRID.
July 16, 1982. The CDC releases a Morbidity and Mortality Weekly Report (MMWR) detailing a rare pneumonia amongst three hemophiliacs. Publication of this report prompted a meeting 11 days later, bringing together representatives from the Food and Drug Administration (FDA), CDC, Public Health Service, National Institutes of Health, American Blood Resources Association, and National Hemophilia Foundation.
December 1982. An infant who had received a transfusion dies of an “immune deficiency illness.” In the MMWR report outlining the infant’s death, the CDC also reports first AIDS cases among people with hemophilia and “expresses concern about the possible transmission of AIDS through blood and blood products.”
January 4, 1983. The CDC hosts a meeting to address the growing concern over the safety of the nation’s blood supply. At this meeting, CDC official Don Francis demanded, “How many people [with hemophilia] have to die? Is three enough? Is six? Is ten? Is a hundred enough? Just give us the number so we can set the threshold!” Yet the meeting produces no recommendations or changes. Coming back from the conference, Don Francis wrote that “for hemophiliacs I fear it might be too late…1/3 to 1/2 of hemophiliacs might already be exposed.”
March 1983. FDA begins approving licensing for heat treatment of clotting factor. Several US manufacturers begin the development of heat-treated factor concentrate after testing shows the process kills both the HIV and hepatitis viruses.
The CDC issues another MMWR stating that AIDS was being transmitted through infected blood and blood products, placing the entire bleeding disorders community and those who use blood products at risk of infection. The CDC recommends steps to be taken immediately to keep the blood supply safe.
May 11, 1983. The National Hemophilia Foundation sends notices to individuals and local chapters urging patients to continue the use of clotting factor, despite warnings from the CDC that the blood supply is tainted with HIV/AIDS.
October 1983. Manufacturers begin issuing clotting factor recalls.
January 1984. Based on a report from the Annals of Internal Medicine, transfusion-associated HIV/AIDS is proven to be sexually transmittable. Community members begin to consider how their HIV/AIDS status would impact their close relationships.
March 1984. The first diagnostic test for HIV, the retrovirus that causes AIDS, is developed. The number of people with hemophilia diagnosed with HIV/AIDS is growing rapidly.
October 1984. The CDC reports an alarmingly high number of factor concentrates contain HIV/AIDS.
How many dead hemophiliacs do you need?
Don Francis
January 4, 1983
1985. In the midst of fear and confusion, Ryan White and his family from Indiana emerge as spokespersons for those living with hemophilia and HIV/AIDS, especially after Ryan is denied entry to his school.
The FDA stops issuing licenses for non-heat-treated clotting factor, however, the decision is left up to manufacturers on how to handle their inventory. It is 1989 before non-heat-treated clotting factor is formally subject to recall and destruction.
April 1985. Letters from the National Hemophilia Foundation continue to reaffirm the recommendation that patients use clotting factor even as product recalls are issued.
1986. The first product for the treatment of von Willebrand Disease becomes commercially available.
1989. During this period, many in the hemophilia community feel they are not getting the answers they seek. The Committee of Ten Thousand (COTT) and Hemophilia-HIV/PEER Association are formed to demand more information and transparency for those who contracted HIV/AIDS from tainted blood product.

Like Ryan White, three hemophiliac brothers are denied entry to their Florida school. The Ray brothers take their case to court and are awarded the right to return to school. Shortly after the decision, their home burns to the ground, almost certainly as a result of arson. The media coverage following the fire is often considered a significant event in the history of HIV/AIDS in the United States.
April 17, 1989.
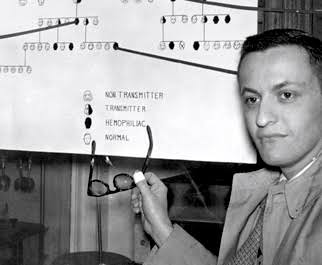
The 90s
1990. Ryan White dies at the age of 18. Inspired by Ryan’s advocacy, Congress passes the Ryan White CARE Act in his honor later that year, providing federal assistance to people with HIV who would otherwise struggle to afford treatment.
1992. The hemophilia patient community continues to galvanize. Local and national hemophilia organizations and others seek answers to why they were given misinformation and to hold accountable those who failed to prevent the spread of HIV/AIDS to people with hemophilia.
Dr. Dana Kuhn amasses 350 documents (“The Trail of AIDS”) detailing the sequence of events on how inadequate decision-making led to the widespread infection of HIV/ AIDS among the hemophilia patient community.
Blood screening for hepatitis C becomes available.
The first recombinant factor VIII concentrate becomes commercially available. It is 1997 before a recombinant factor IX concentrate becomes commercially available. Recombinant products are not made from human plasma and so do not carry the same risk for transmitting diseases such as HIV/AIDS and hepatitis.
Ricky Ray, one of three brothers infected with HIV/AIDS, dies at age 15.
1994. Litigation against drug manufacturers dominates most of the 1990s. Some patients file individual cases, while others are part of large class-action suits. Others choose not to pursue a settlement.
1994
Hemophilia Federation of America forms as a sub-group of COTT in 1993 and becomes independent in 1994. HFA bridges the gap between the advocacy efforts COTT is working on, and is a place of education, advocacy, and awareness for families living with bleeding disorders.



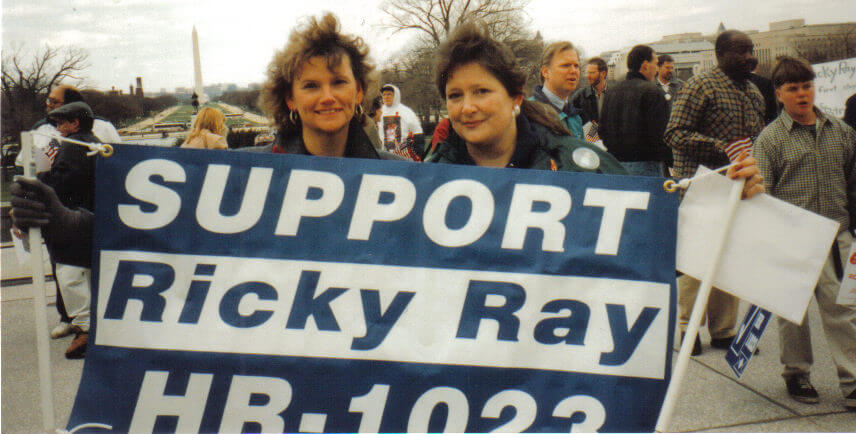

1995. Following a request from the Department of Health and Human Services, a committee of the Institutes of Medicine (IOM) reviews the scientific evidence that was available to decision makers during the early 1980s when the HIV/AIDS epidemic first emerged. The IOM releases a report called, HIV and the Blood Supply: An Analysis of Crisis Decision Making, which outlines several recommendations for making the blood supply safer and gives a list of action items including the need for consumer representation on governmental blood advisory groups. The report is viewed by some in the community as a turning point on blood safety and patient engagement.
Many of the HIV/AIDS infected seek compensation due to the mounting medical debt and demand accountability from the government and manufacturers. Newly diagnosed families worry that the lawsuits could potentially keep them from accessing newer, safer clotting factor.
1996. While other countries are finalizing settlements by 1996, negotiations in the U.S. continue due to years of stalled litigation. After the IOM report and many attempts at class action suits, some victims and their families begin to gain traction in holding the pharmaceutical companies accountable for infected clotting factor products. The financial burden of lost loved ones is felt by their families.
1997. Advocates mobilize the community in support of the Ricky Ray Hemophilia Relief Fund Act. As a result of the community’s tireless grassroots efforts, Congress begins to show support for those affected.
1998. After tireless efforts on the part of the community, President Clinton signs the Ricky Ray Hemophilia Relief Fund Act of 1998 on November 12, 1998. The legislation provides for “compassionate payments” of $100,000 to individuals who contracted HIV due to contaminated clotting factor.
The first day of camp was exciting because seeing a friend again meant they had survived, and sad because the missing faces from last year’s cabin meant those friends had died.
Jeff Johnson
The 2000s
2000-2005. The hemophilia community sees a resurgence in families coming together through camps and local and national meetings. The term “hemophilia” begins to be replaced by the term “bleeding disorders” to acknowledge all bleeding conditions. Treatment costs continue to rank among the most expensive chronic conditions. The financial impact continues to burden families.
2001. Recombinant factor VIII product shortages instill concern about the supply of necessary clotting factor concentrates.
2003. Attention is now given to other rare bleeding disorders, the newly diagnosed, women, people of color, and those living in rural areas.
2006. Although about 30% of hemophilia A and 2-3% of hemophilia B patients will form an inhibitor, and inhibitors have been of concern since clotting factor was discovered in the 1960s, resources and education about this complication was scarce. Education and outreach increase for inhibitor families in the mid-2000s.
2007. Dr. Marilyn Manco-Johnson et al., publish a multi-year study showing a prophylactic regimen of treatment prevents joint damage in pediatric patients in the New England Journal of Medicine.
2008. Lifetime caps on insurance coverage persist, raising concern in the hemophilia community— especially for inhibitor patients. This issue will later inform decisions around the Affordable Care Act.
2009. A product for those with factor I deficiency becomes commercially available.
May 21, 2008
President George W. Bush signs into law the Genetic Information Nondiscrimination Act (GINA) to protect Americans against discrimination based on their genetic information when it comes to health insurance and employment.



The 2010s
2010. The Affordable Care Act passes and is signed into law by President Barack Obama. The ACA addresses lifetime caps, pre-existing conditions, out-of-pocket expenses, young adult coverage, and other needs important to people with bleeding disorders.
2011. Hepatitis C continues to devastate the bleeding disorders community and is the leading cause of death in adults with hemophilia. This is the defining issue for the adult bleeding disorders population; available treatments are difficult and often not effective.
The National Hemophilia Foundation, in partnership with others, launches My Life, Our Future, a project to genotype hemophilia patients and carriers.
A product for factor XIII deficiency becomes commercially available.
2013. The decade sees continued improvements in and expansion of research, education, and conversation about von Willebrand disease and women with bleeding disorders.
After years of continued struggle with hepatitis C, a new medicine becomes commercially available. Hepatitis C is no longer the leading cause of death in adult hemophiliacs in the U.S. The new product is hailed as a cure for hepatitis C.
2014. Extended half-life clotting factor VII and IX concentrates become commercially available.
2015. The first recombinant product for von Willebrand Disease becomes commercially available. The first factor X product becomes commercially available.
After 32 years of an FDA ban on blood donations from men who have sex with men (MSM), the FDA reconsiders and votes to modify the ban to defer blood donations by MSM and their female partners for one year after last sexual contact.
2015-2016. Opioid dependency increasingly becomes a topic of concern for the bleeding disorders community.
2017. The National AIDS Memorial Grove unveils a dedicated area and memorial for the hemophilia community.
With a new administration in place, Congress makes multiple attempts to dismantle the Affordable Care Act. In a floor speech, Senator Bernie Sanders asserts that the hemophilia community would be disproportionately impacted. The repeal efforts fail.
A subcutaneous, bi-specific antibody therapy becomes commercially available to those with hemophilia A and inhibitors. This product gets approval for hemophilia A patients a year later.
2019. Since 2011, more than 19 new products for the treatment of bleeding disorders have become commercially available.